
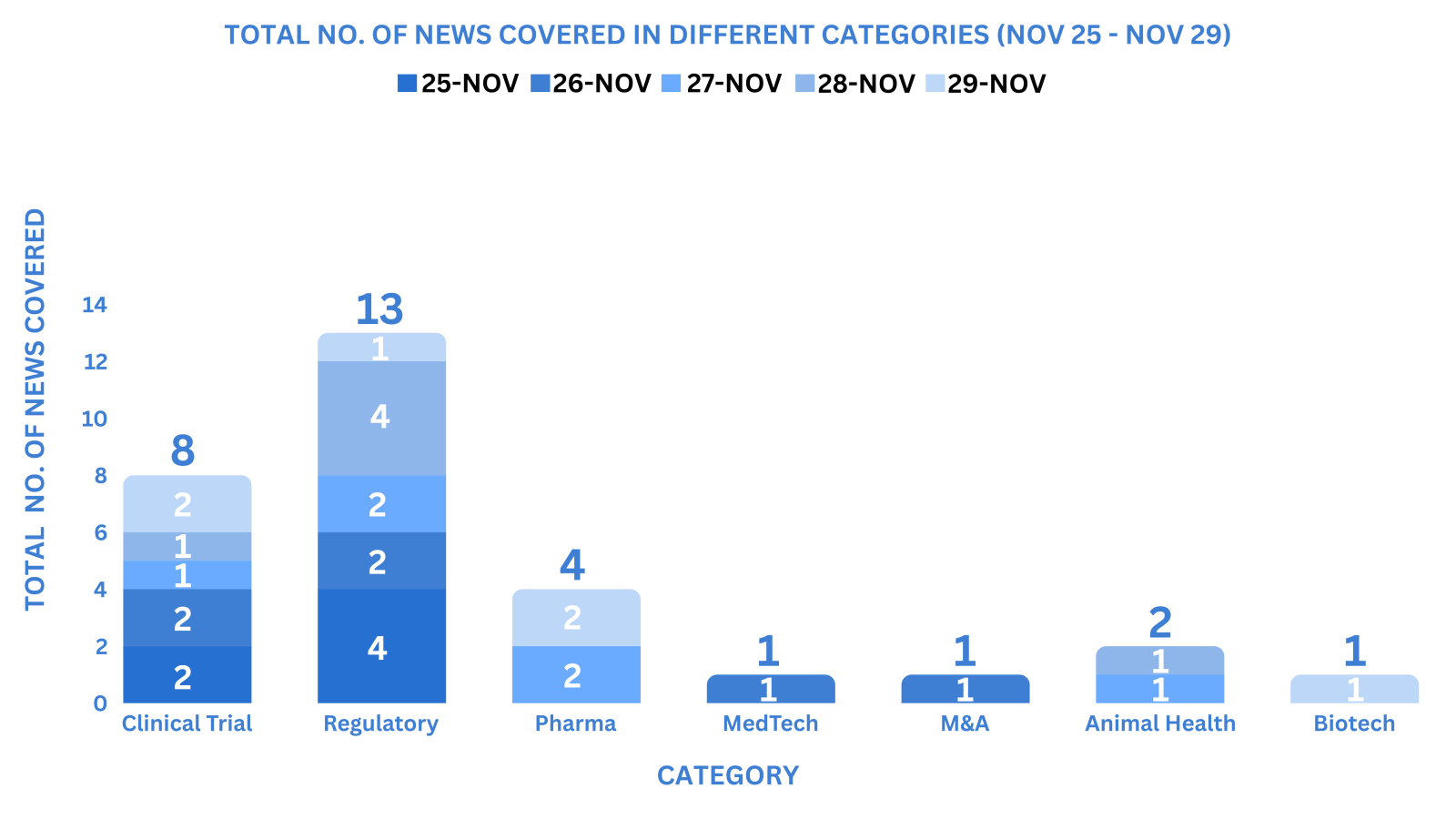
PharmaShots Weekly Snapshots (November 25 – November 29, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Animal Health & Biotech. Check out our full report below:

AstraZeneca Provides Update on P-III (CAPItello-281) Study of Truqap Regimen in PTEN-Deficient mHSPC
Read More: AstraZeneca
Orbus Therapeutics Reports the P-III (STELLAR) Trial Data of Eflornithine Plus Lomustine to Treat Recurrent Astrocytoma IDH Mutant Grade 3
Read More: Orbus Therapeutics
Merck Reports Results from the P-III (ZENITH) Study of Winrevair (Sotatercept-csrk) for Pulmonary Arterial Hypertension
Read More: Merck
MaaT Pharma Reports Results from the P-Ib (IASO) Study of MaaT033 for Treating Amyotrophic Lateral Sclerosis (ALS)
Read More: MaaT Pharma
Amgen Reports 52 Weeks Data from P-II Trial of Maritide in Obese People with or without Type 2 Diabetes
Read More: Amgen
Telix Pharmaceuticals Reports the First Patient Dosing in P-III (ZIRCON-CP) Trial of TLX250-CDx for Kidney Cancer Imaging in China
Read More: Telix Pharmaceuticals
Galderma Publishes the P-III (OLYMPIA 1) Trial of Nemolizumab for Prurigo Nodularis in JAMA Dermatology
Read More: Galderma
UroGen Pharma Reports Long-Term Data from OLYMPUS Trial of Jelmyto for Upper Tract Urothelial Cancer
Read More: UroGen Pharma

Merck’s Welireg (Belzutifan) Receives Chinese Approval to Treat Certain Types of Von Hippel-Lindau (VHL) Disease-Related Tumors
Read More: Merck
GSK Reports the US FDA’s BLA Acceptance of Blenrep (Belantamab Mafodotin) Regimen for Treating R/R Multiple Myeloma
Read More: GSK
Johnson & Johnson Submits sBLA to the US FDA for Subcutaneous Induction Regimen of Tremfya (Guselkumab) for Ulcerative Colitis
Read More: Johnson & Johnson
StemCyte Reports the US FDA’s Approval of Regenecyte Cord Blood Cell Therapy Product
Read More: StemCyte
Alnylam Pharmaceuticals Reports the US FDA’s sNDA Acceptance of Amvuttra (Vutrisiran) to Treat ATTR Amyloidosis with Cardiomyopathy
Read More: Alnylam Pharmaceuticals
Intellia Therapeutics’ Nexiguran Ziclumeran Secures the US FDA’s RMAT Designation to Treat Hereditary Transthyretin (ATTR) Amyloidosis with Polyneuropathy
Read More: Intellia Therapeutics
Novartis’ Kisqali (Ribociclib) Secures EC’s Approval as an Adjuvant Treatment of HR+/HER2- Early Breast Cancer
Read More: Novartis
GSK’s Menveo Secures the EC’s Approval to Prevent Invasive Meningococcal Disease
Read More: GSK
BeiGene’s (to be BeOne Medicines) Tevimbra Secures the EC’s Approval as a 1L Treatment of Esophageal Squamous Cell Carcinoma and G/GEJ Cancer
Read More: BeiGene
Lin BioScience’s LBS-007 Secures the US FDA’s Fast Track Designation to Treat Acute Myeloid Leukemia
Read More: Lin BioScience
Applied Therapeutics Receives Complete Response Letter for Govorestat’s NDA for Treating Classic Galactosemia
Read More: Applied Therapeutics
AOP Health’s Rapiblyk (landiolol) Receives the US FDA’s Approval for Atrial Fibrillation and Atrial Flutter in the Critical Care Setting
Read More: AOP Health
CervoMed’s Neflamapimod Secures the US FDA’s Orphan Drug Designation to Treat Frontotemporal Dementia
Read More: CervoMed

Acadia Pharmaceuticals Collaborates with Saniona to Develop and Commercialize SAN711
Read More: Acadia Pharmaceuticals and Saniona
Arrowhead & Sarepta Sign a Global Licensing and Collaboration Agreement for Multiple Clinical and Preclinical Programs
Read More: Arrowhead & Sarepta
Allink Biotherapeutics Secures $42M to Support the Development of Bispecific Antibody and ADC Pipeline
Read More: Allink Biotherapeutics
Formosa and Medvisis Partner for Clobetasol Propionate Ophthalmic Suspension to Treat Post-Operative Inflammation and Pain
Read More: Formosa and Medvisis

BrioHealth Solutions Initiates Patient Recruitment in INNOVATE Trial of BrioVAD System for Advanced Heart Failure
Read More: BrioHealth Solutions

Roche to Acquire Poseida Therapeutics for ~$1.5B
Read More: Roche and Poseida Therapeutics

Merck Animal Health Receives the EC’s Approval of BRAVECTO TriUNO for Use in Dogs
Read More: Merck Animal Health
Boehringer Ingelheim Introduces Vetmedin Solution (Pimobendan Oral Solution), for Congestive Heart Failure in Dogs
Read More: Boehringer Ingelheim

SELLAS Reports Data from Preclinical Studies Suggesting ASXL1 Mutations as Predictor of SLS009 Response in Solid Tumors
Read More: SELLAS
Related Post: PharmaShots Weekly Snapshots (November 18 – November 22, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














